half life formula calculus
Calculate the half life of. N t N 0 e -tτ N t N 0 e -λt τ is the.

Solving Half Life Problems With Exponential Decay Krista King Math Online Math Help
Approximate Half-Life and Doubling-Time.

. The half-life eqt_ 12 eq of a substance with decay constant eqlambda eq can be calculated as eqt_ 12 dfrac ln 2 lambda eq seconds. T_frac 12 frac ln2 k where k is the decay constant. So when were dealing with.
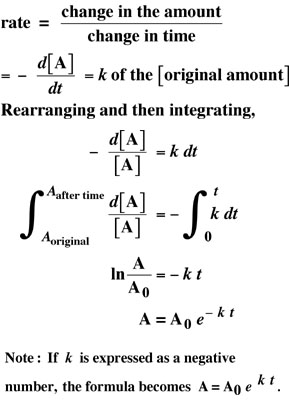
The Formula for Half-Life We can describe exponential decay by the following given decay equation. Then A 800 12 300006000 800 12 5 800 05 5 800003125 25. N t the quantity that still remains and has not yet decayed after a time t.
Putting this value in the above equation. It is also possible to determine the remaining quantity of a substance using a few other parameters. Where t 12 is the half-life of the particle t is the elapsed time N 0 is the quantity in the beginning and.
Log e N log e N o λt. The formula for the half-life is obtained by dividing 0693 by the constant λ. Here λ is called the disintegration or decay constant.
The general equation with half life. Hence the formula to calculate the half-life of a substance is. T12 is the half-life.
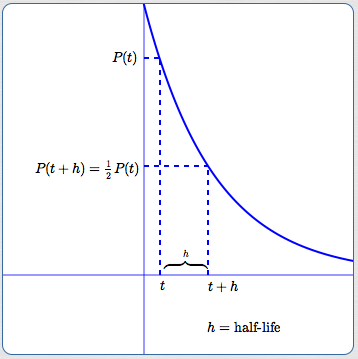
Half-life is the time required for the amount of something to fall to half its initial value. T is the half-life. We know that there is.
They give us the initial amount but we dont need it to determine k. Sometimes we know the percent change r in ftAcdot1rt but were interested in the half-life or doubling time. For half-life we use the equation At A_o ekt.
The formula for the half-life is obtained by dividing 0693 by the constant λ. N t N 0 05 t T. Thus N N o 2.
You can find the half-life of a radioactive element using the formula. The mathematical representation of Half life is given by Half. Log e NN o λt i At half-life the value of N reduces to half of the initial value.
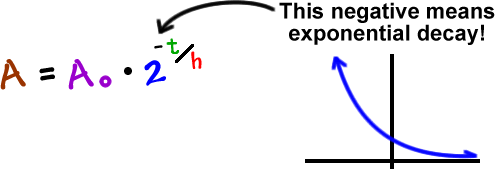
Below are shown three equivalent formulas describing exponential decay. To calculate the half-life we want to know when the quantity reaches half its original size. T 12 the half-life of the decaying quantity.
The time required for the original quantity to decay to half its amount is called the half-time. So when were dealing with half life specifically instead of exponential decay in general we can use this formula we got from substituting y c 2 yc2 y c 2. In which N 0 is the number of atoms you start with and N t the number of atoms left after a certain time t for a.
Nt is the remaining quantity after time t. A P12 td. Half-life means that half of the material is gone in 1690 years.
N 0 the initial quantity of the substance that will decay. τ is the mean lifetime. Half-Life Decay Formula.
So 25 g of carbon-14 will. It is the time requires to decay in half. N0 is the initial quantity.
So we can substitute this value in for y and then simplify the decay formulafracC2Cektfrac12ekt. Learn the formula for half life as well as see an example in this free math video tutorial by Marios Math Tutoring009 Formula for Calculating Half Life03.

How To Find Half Life Algebra Study Com
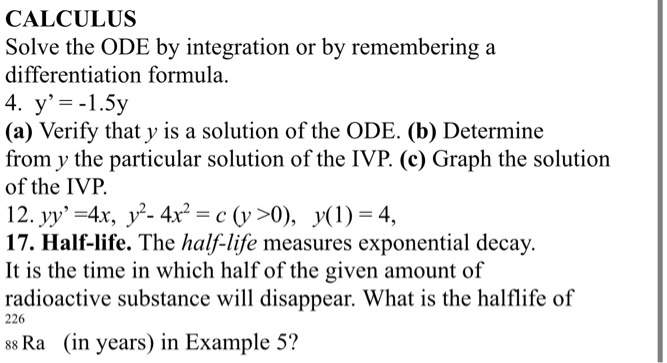
Solved Calculus Solve The Ode By Integration Or By Remembering A Differentiation Formula Y Sy A Verify That Y Is A Solution Of The Ode B Determine From Y The Particular Solution
How To Solve Half Life Problems In Calculus Quora
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels

Ch 6 4 Exponential Growth Decay Calculus Graphical Numerical Algebraic By Finney Demana Waits Kennedy Ppt Video Online Download
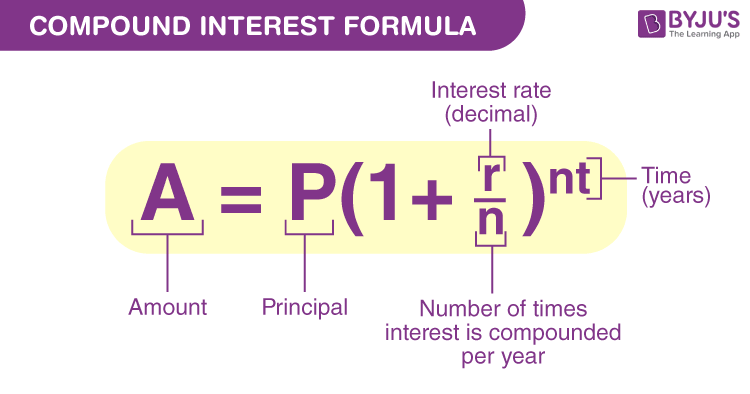
Compound Interest Definition Formulas And Solved Examples
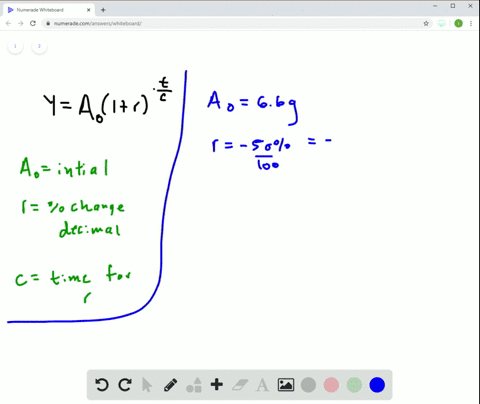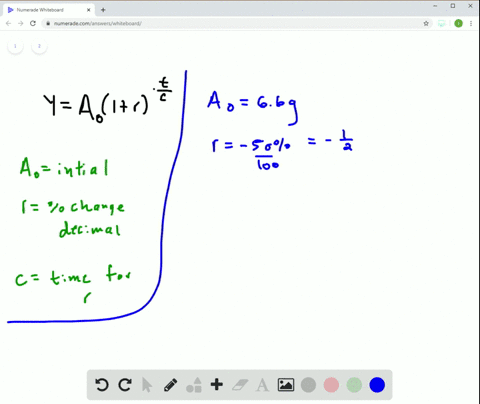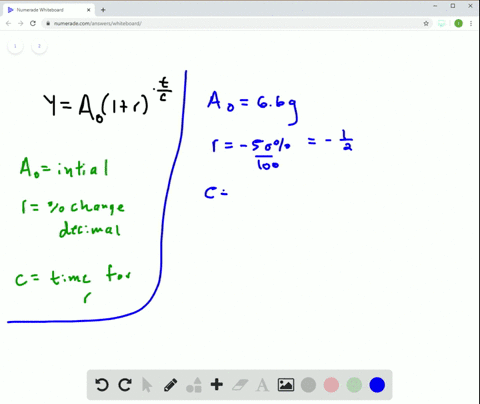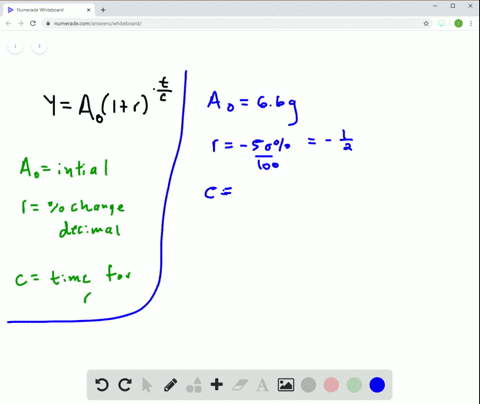
Solved Radioactive Decay The Half Life Of Phosphorus 32 Is About 14 Days There Are 6 6 Grams Present Initially A Express The Amount Of Phosphorus 32 Remaining As A Function Of Time T B When

Precalculus Spring Half Life Problem Youtube
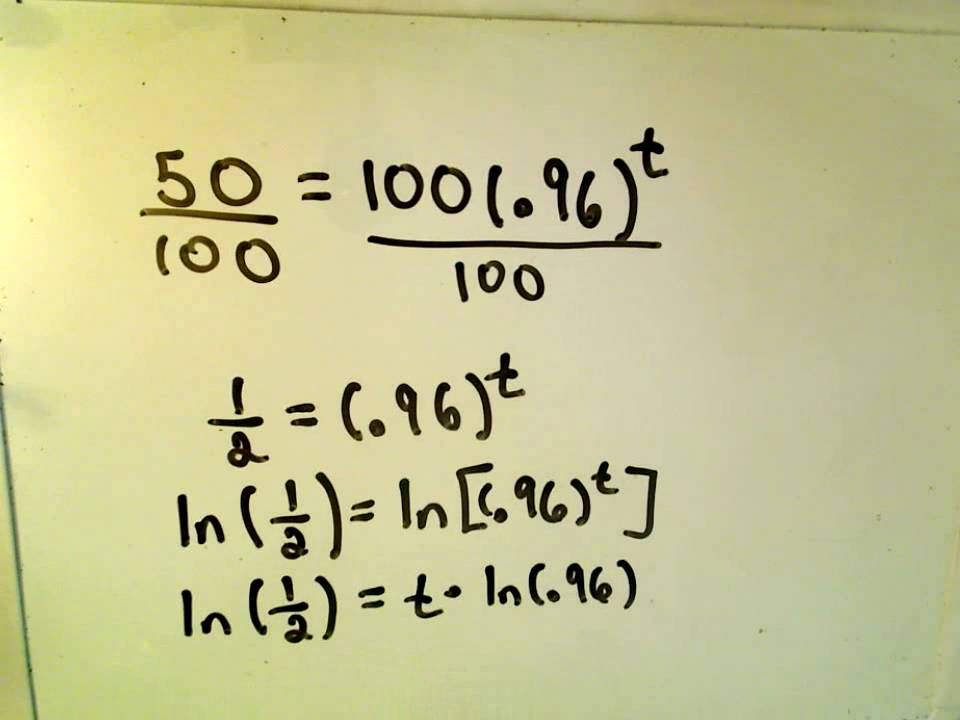
Exponential Decay Finding Half Life Youtube
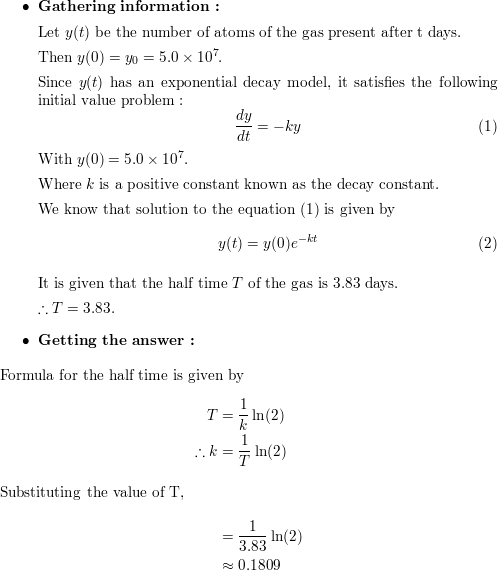
Radon 222 Is A Radioactive Gas With A Half Life Of 3 83 Days Quizlet

Doubling Time And Half Life Of Exponential Growth And Decay Math Insight

How To Calculate Half Life Of A First Order Reaction Chemistry Study Com